Prof. dr. Jeroen Pasterkamp

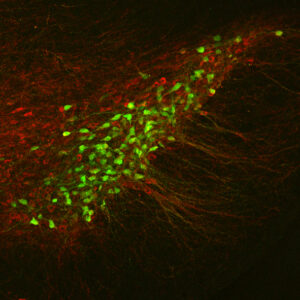
 One of the most challenging problems in biology is to understand how the billions of neurons in the mammalian nervous system “wire up” to form functional neural circuits that underlie all behavior. This has been one of the most intensely studied areas of developmental neurobiology in the past, and a number of important proteins have been identified that instruct axons to project to their specific target regions (so called axon guidance proteins). Our current work focuses on how axon guidance proteins are detected by receptor proteins on growing axons, how these receptors signal and how they are regulated. Remarkably, our nervous system contains billions of connections but no more than a hundred axon guidance proteins. How can this relatively small number of proteins set up the wiring of a disproportionally large number of connections with many different characteristics such as trajectories or synaptic partners? Evidence is emerging that the effects of axon guidance proteins are diversified and spatiotemporally controlled, vastly expanding the number of guidance decisions they can mediate.
One of the most challenging problems in biology is to understand how the billions of neurons in the mammalian nervous system “wire up” to form functional neural circuits that underlie all behavior. This has been one of the most intensely studied areas of developmental neurobiology in the past, and a number of important proteins have been identified that instruct axons to project to their specific target regions (so called axon guidance proteins). Our current work focuses on how axon guidance proteins are detected by receptor proteins on growing axons, how these receptors signal and how they are regulated. Remarkably, our nervous system contains billions of connections but no more than a hundred axon guidance proteins. How can this relatively small number of proteins set up the wiring of a disproportionally large number of connections with many different characteristics such as trajectories or synaptic partners? Evidence is emerging that the effects of axon guidance proteins are diversified and spatiotemporally controlled, vastly expanding the number of guidance decisions they can mediate.
However, the molecular details of this regulation and its relevance for vertebrate axon guidance in vivo remain incompletely understood. Another aim of our work is therefore to resolve key molecular events that enable a limited number of axon guidance proteins to dictate a vast number of wiring decisions in vivo. This work is in part supported by a VICI grant from ALW-NWO and the NWO Gravitation Program BRAINSCAPES.
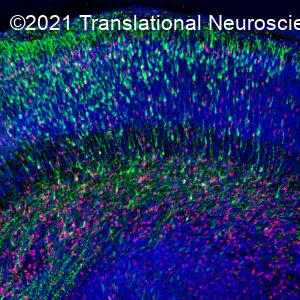
 Many brain nuclei can be further subdivided into smaller neuronal subsets with specific molecular signatures and functions. For example, the midbrain dopamine system is composed of the ventral tegmental area (VTA) and the substantia nigra (SN). Both parts have different functions and are differentially affected in disease (SN neurons die in Parkinson disease patients). However, even within the VTA and SN smaller neuronal subsets exist. We are currently setting up subtractive genetics strategies in mice to genetically label, purify, activate or ablate functionally similar subsets of neurons in the dopamine system and other parts of the nervous system such as the habenula. Using this method, we have identified many aspects of dopaminergic pathway development (e.g. rosto-caudal extension, axonal orientation, fasciculation). This new approach will help us to study novel aspects of dopaminergic pathway development such as axonal pruning, topographic bundle organization and axonal sorting. This work is in part supported by Stichting ParkinsonFonds and the NWO Gravitation Program BRAINSCAPES.
Many brain nuclei can be further subdivided into smaller neuronal subsets with specific molecular signatures and functions. For example, the midbrain dopamine system is composed of the ventral tegmental area (VTA) and the substantia nigra (SN). Both parts have different functions and are differentially affected in disease (SN neurons die in Parkinson disease patients). However, even within the VTA and SN smaller neuronal subsets exist. We are currently setting up subtractive genetics strategies in mice to genetically label, purify, activate or ablate functionally similar subsets of neurons in the dopamine system and other parts of the nervous system such as the habenula. Using this method, we have identified many aspects of dopaminergic pathway development (e.g. rosto-caudal extension, axonal orientation, fasciculation). This new approach will help us to study novel aspects of dopaminergic pathway development such as axonal pruning, topographic bundle organization and axonal sorting. This work is in part supported by Stichting ParkinsonFonds and the NWO Gravitation Program BRAINSCAPES.
Non-coding RNAs are important players in neural development and brain disease. We focus on different classes of non-coding RNAs: microRNAs (miRNAs), circular RNAs (circRNAs) and transfer RNA fragments (tRFs).
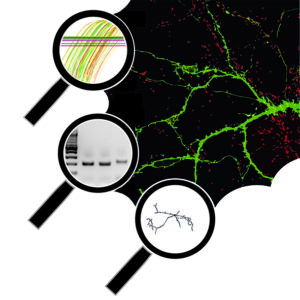 MiRNAs are small non-coding RNAs (22 nt) that bind specific mRNA targets thereby regulating their translation and stability. Deregulation of miRNAs has been implicated in various brain disorders and these molecules are considered to be promising therapeutic targets in many of these disorders. Our previous work shows that miRNAs are deregulated at different levels in patients suffering from temporal lobe epilepsy (TLE) and using a multidisciplinary approach (e.g. RNA-seq, biotin-IP, in vivo Ago IPs, epilepsy models, etc.) we aim to decipher how miRNAs contribute to the process of epileptogenesis and how these effects can be reversed.
MiRNAs are small non-coding RNAs (22 nt) that bind specific mRNA targets thereby regulating their translation and stability. Deregulation of miRNAs has been implicated in various brain disorders and these molecules are considered to be promising therapeutic targets in many of these disorders. Our previous work shows that miRNAs are deregulated at different levels in patients suffering from temporal lobe epilepsy (TLE) and using a multidisciplinary approach (e.g. RNA-seq, biotin-IP, in vivo Ago IPs, epilepsy models, etc.) we aim to decipher how miRNAs contribute to the process of epileptogenesis and how these effects can be reversed.
Circular RNAs are a (re)discovered class of long non-coding RNAs with a circular structure. Besides being very stable due to their circular structure, circRNAs are enriched in the brain and in specific subcellular compartments. Most studied functions include miRNA and RNA binding protein (RBP) sponging, yet, the involvement of circRNAs in different molecular processes remains to be determined. We aim to determine which circRNAs are important for axonal development and how they affect this process. Similarly, we study deregulated circRNAs in neurological diseases, such as refractory epilepsy.
Fragments derived from tRNAs are implicated in different aspects of gene regulation. One example is their miRNA-like function. We are interested in finding out how these fragments contribute to brain disease.
Further, we are constantly working on technical approaches to detect, visualize and manipulate non-coding RNAs, both in vitro and in vivo.
Our research is in part supported by the Epilepsiefonds and the Marie Curie ITN circRTrain. Furthermore, part of this work is performed in close collaboration with InteRNA Technologies.
Amyotrophic lateral sclerosis (ALS) is a devastating neurological disease affecting roughly 50,000 people at any time in Europe, and causing around 10,000 deaths each year. It can occur in any individual at anytime during adulthood. ALS is characterized by progressive degeneration of motor neurons in brain and spinal cord leading to muscle weakness. Initial manifestations are weakness of limbs or weakness in the bulbar region leading to abnormalities of speech, swallowing difficulties and facial weakness. The patient progressively becomes paralyzed and dies eventually as a result of respiratory failure. There is no cure for ALS. The only available drugs are marginally effective in extending the life span of ALS patients with a few months.
Novel treatment options are needed for this disabling and fatal disease. The lack of treatment for ALS can be attributed to an absence of validated therapeutic targets reflecting an inadequate understanding of disease mechanisms. Elucidating the ALS pathogenic mechanism is therefore essential to pave the road for therapeutic interventions. Several projects in the lab are focused on unravelling the molecular mechanisms defective in familial and sporadic ALS.
An important starting point of our work are genetic defects detected in ALS patients (e.g. detected in Project MinE). Using different molecular cell biological, -omics, and biochemical approaches in different model systems (e.g. IPSC-generated neurons or organoids) we aim to understand how specific genetic defects cause ALS and on basis of this knowledge design therapeutic strategies. This work is in part supported by Stichting ALS Nederland and H2020 (E-Rare grants “INTEGRALS” and MAXOMOD”).
In addition to ALS, we also study other neurodegenerative disorders, including Alzheimer’s and Parkinson’s disease, in part with support from Stichting ParkinsonFonds and the JPND project ‘TRIAGE’. We use similar models and approaches to dissect disease mechanisms for these disorders and for designing therapeutic approaches.
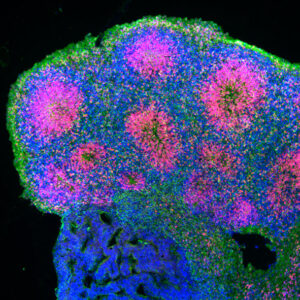
Furthermore, genome editing approaches, such as CRISPR/CAS9, are used to generate isogenic controls or cell lines carrying specific mutations. iPSCs are also used to generate various types of brain organoids allowing analysis of neuronal and glial cell morphology and function in a three-dimensional environment, for example for studying ALS and Alzheimer’s disease. Analysis of neuronal morphology is facilitated by Ultramicroscope Lightsheet imaging present in our lab.
The IPSC- and organoid lab and lightsheet imaging platform is organized in a research facility named the MIND facility. This facility is financially supported by the UMC Utrecht Brain Center, UMC Utrecht and Utrecht University, and managed by the Pasterkamp lab.